Quotations
Thought for Us
Imagination takes you everywhere
Invitation to serve as MRS session chair

Thank you for the opportunity to present:
"Near-field Heat Transfer by Quantum Mechanics"
at the November Meeting.
However, I have a problem!!!
A few days ago, I was informed of having prostrate cancer.
Therefore, I am requesting that my live PPT presentation be a Vimeo video.
https://vimeo.com/642645515
Just enter the URL in a PC browser online. Have Zoom show you how to share during meeting.
I would be available in-person to answer questions.
Thomas Prevenslik
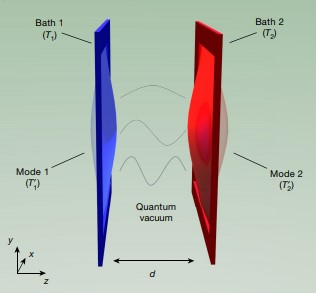
Near-Field Radiative Heat Transfer
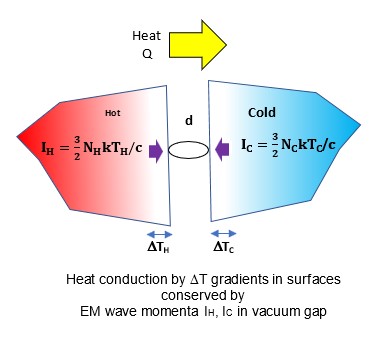
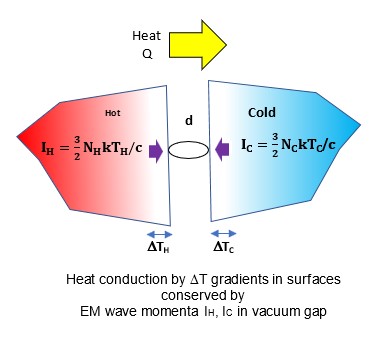
Near-fiield heat radiative transfer (NFRHT) is based on the classical Fluctuation Dissipation Theorem that requires the heat produced in macroscopic bodies upon absorbing EM radiation creates temperature fluctuations that is reciprocated by temperature fluctuations producing EM radiation. But NFRHT in nanoscale gaps between hot and cold bodies is governed by quantum mechanics (QM) and not classical physics. Indeed, the Planck law precludes temperature fluctuations in nanoscale gaps upon absorbing heat. However, all known NFRHT theories are based on the difference of surface temperatures of nanoscale gaps. In effect, the Planck law requires theories of NFRHT that do not depend on temperature which means all known NFRHT theories are invalid.
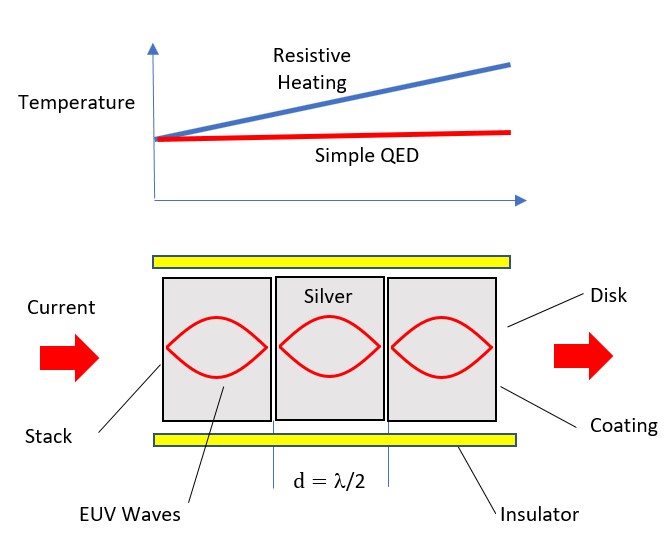
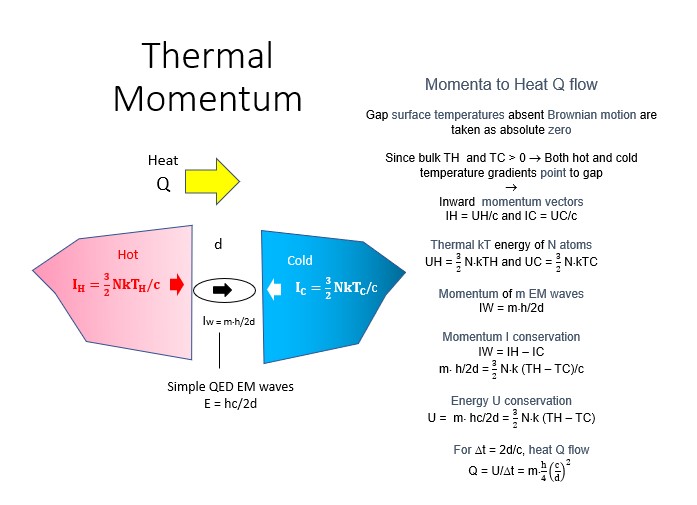
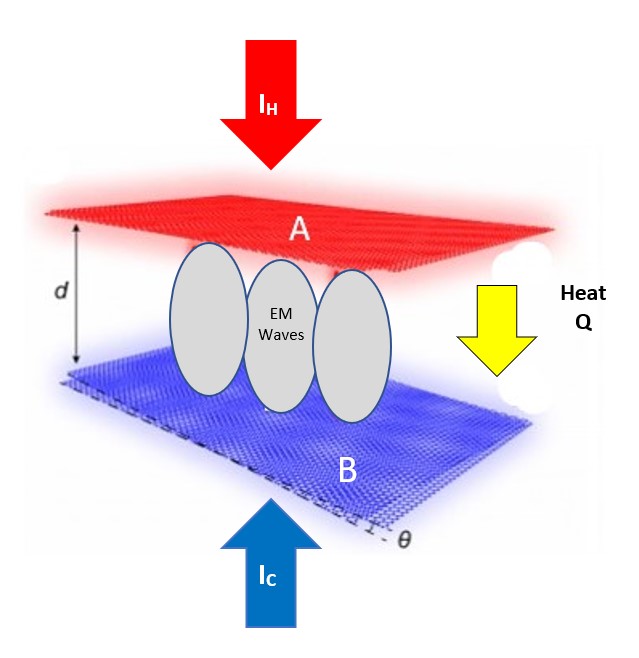
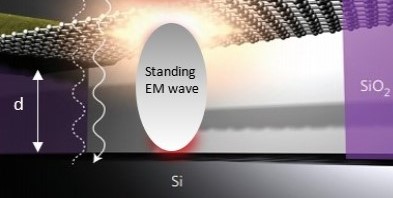
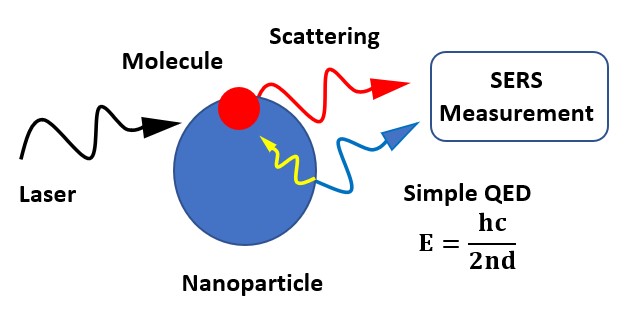
One temperature independent NFRHT theory is simple QED based on the Planck law that conserves heat by creating temperature independent EM standing or travelling waves across the evacuated nanoscale gaps. But the heat requires EM confinement across the gap to a half-wavelength having the dimension d of the gap. In simple QED, the EM confinement is provided by the momenta of the thermal energy of the in the heat flow between hot and cold bodies, the hot and cold momenta directed toward their gap surfaces because of the thermal gradient formed with gap surface temperatures at absolute zero, the absolute zero temperatures because the Planck law requires Brownian motion to cease. Heat flows if the hot momentum exceeds the cold momentum.
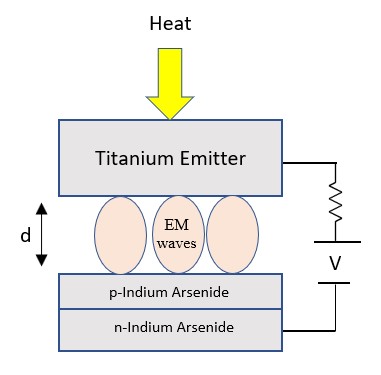
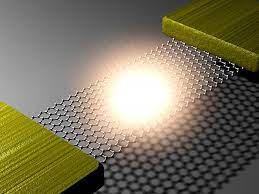
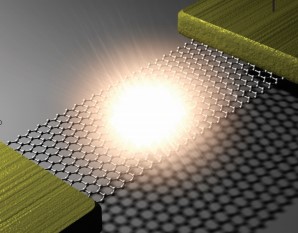
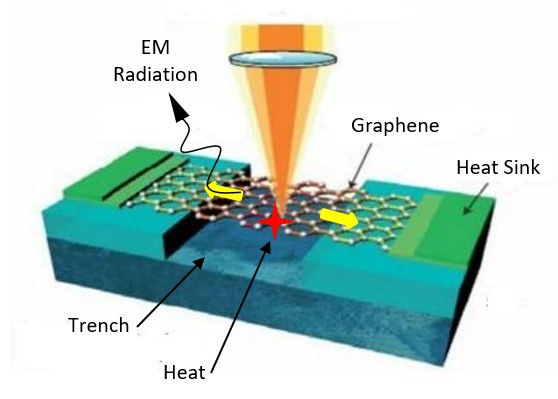
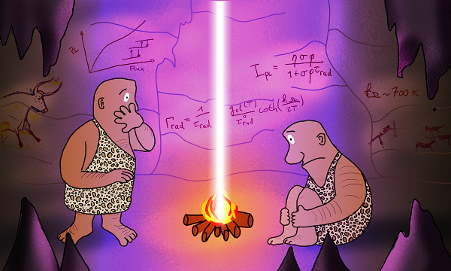
Simple QED highlights the stark difference between QM and classical physics illustrated by the emission of bright white-light from a Joule heated single graphene layer (0.337 nm) over a nanoscale trench reported in the literature.
The application of NFRHT to the nanoscale trench is difficult to implement as the Joule heat from the graphene layer into the nanoscale trench is not known. Instead, the Fourier equation was applied to the graphene layer assuming Joule heating produces electron temperatures of 2800 K giving graphene temperatures near 2000 K. But bright-white light may require higher temperatures, say up to 5000 K. In contrast, simple QED argues the graphene remains at ambient temperature. Instead, 1.8 keV soft X-rays are produced across the graphene thickness that fluoresce down to about 200 eV, the heat of which is either lost to the surroundings or enters the trench, the latter by simple QED creating IR waves standing in the trench, the overtones of the IR producing the observed bright white-light absent high temperatures. Consistent with simple QED, the graphene does not acquire 2800 K electron temperatures evidenced by the observation of white-light at 10 K. Indeed, there is a stark difference between QM and classical physics and should be expected whenever classical physics including NFRHT is used to explain observations at the nanoscale.
See Video
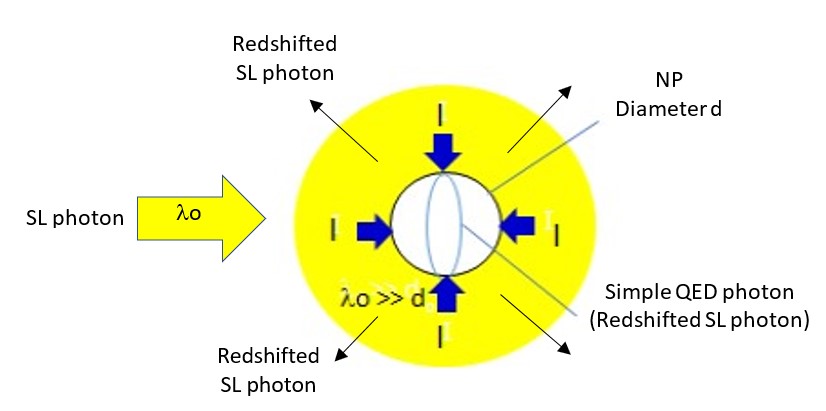
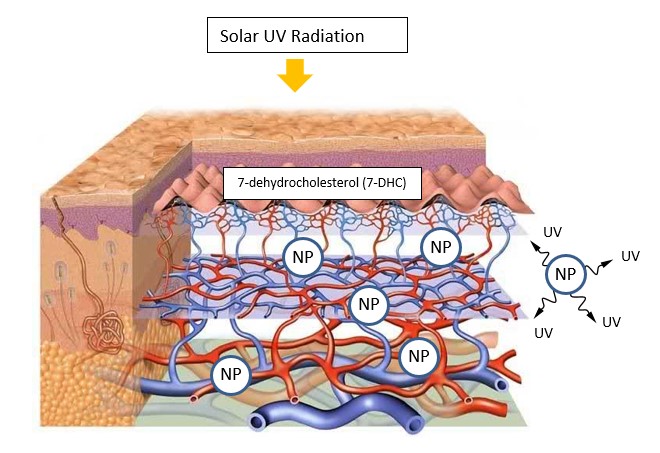

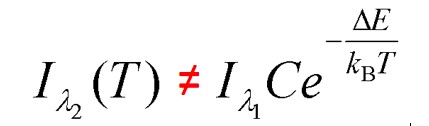



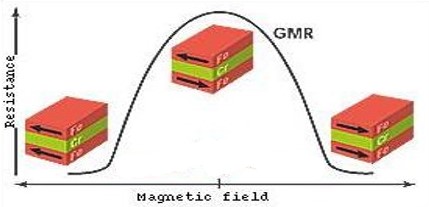




Summary argument:
Since influenza vaccines based on inactivated virus have only 50% efficacy, and since Moderna's Covid-19 mRNA vaccine is the genetic equivalent of an inactivated Covid-19 spike, the efficacy of the Covid-19 mRNA vaccine should be near 50% - not over 90%.
Hence,
The mRNA concept with ~ 80 nm lipid NP carriers emitting UVC is unworkable. But the NP Treatment using NP > 100 nm would emit IR which would not inactivate the mRNA to make the mRNA concept viable, but would reduce the Covid-19 efficacy near 50%
The Abstract of 'Nanoparticles and mRNA Covid-19 efficacy", Paper , and mp4 Video are available.
